Description
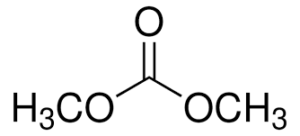
Dimethyl carbonate (DMC) is an organic compound with the formula OC(OCH3)2. It is a colourless, flammable liquid. It is classified as a carbonate ester. This compound has found use as a methylating agent and more recently as a solvent that is exempt from the restrictions placed on most volatile organic compounds (VOCs) in the US. Dimethyl carbonate is often considered to be a green reagent.
Dimethyl carbonate appears as a clear, colorless liquid with a pleasant odor. Denser than water and slightly soluble in water. Vapors are heavier than air. Used to make other chemicals and as a special purpose solvent.|Liquid|COLOURLESS LIQUID WITH CHARACTERISTIC ODOUR.
Dimethyl carbonate appears as a clear, colorless liquid with a pleasant odor. Denser than water and slightly soluble in water. Vapors are heavier than air. Used to make other chemicals and as a special purpose solvent.|Dimethyl carbonate is a carbonate ester that is carbonic acid in which both hydrogens are replaced by methyl groups. A flammable, colourless liquid (m.p. 2-4℃, b.p. 90℃) with a characterstic ester-like odour, it is used as a ‘green’ methylating agent and as a solvent. It has a role as a solvent and a reagent.
Basic Attributes
Molecular Weight:90.0779
Exact Mass:90.0317
EC Number:210-478-4
UNII:KE9J097SPN
ICSC Number:1080
NSC Number:9371
UN Number:1161
DSSTox ID:DTXSID9029192
Color/Form:Colorless liquid
HScode:2920909090
CAS No:616-38-6
Characteristics
PSA:35.53
XLogP3:0.3992
Appearance:colourless liquid
Density:1.069
Melting Point:41674ºC
Boiling Point:90ºC
Flash Point:18ºC
Refractive Index:1.3672-1.3692
Water Solubility:In water, 1.38X10+5 mg/L at 25 °C (est)|Miscible with alcohol and ether|Miscible with acids and alkalies; stable in the presence of water; soluble in most organic solvents|Soluble in oxygenated solvents|Solubility in water: none
Storage Conditions:Flammables area
Vapor Pressure:55.40 mmHg|55.364 mm Hg at 25 °C|Vapor pressure, kPa at 25 °C: 7.4
Vapor Density:Relative vapor density (air = 1): 3.1
Odor:Pleasant odor
OH:3.10e-13 cm3/molecule*sec
Henrys Law Constant:Henry’s Law constant = 6.2X10-4 atm-cu m/mol at 25 °C (est)
Experimental Properties:MP also listed as 4 °C|BP also stated as 89.7 °C|Hydroxyl radical reaction rate constant = 0.4352X10-12 cu cm/molecule-sec at 25 °C (est)
Air and Water Reactions:Highly flammable. Slightly soluble in water.
Reactive Group:Esters, Sulfate Esters, Phosphate Esters, Thiophosphate Esters, and Borate Esters
Reactivity Alerts:Highly Flammable
Reactivity Profile:DIMETHYL CARBONATE reacts with acids to liberate heat along with methanol and carbon dioxide. Strong oxidizing acids may cause a vigorous reaction that is sufficiently exothermic to ignite the reaction products. Heat is also generated by the interaction with caustic solutions. Flammable hydrogen is generated by mixing with alkali metals and hydrides.
Autoignition Temperature:458 °C
Physical Dangers:The vapour is heavier than air and may travel along the ground; distant ignition possible. The vapour mixes well with air, explosive mixtures are easily formed.
Heat of Vaporization:3.8363X10+7 J/Kmol at 273.15 K
Critical Temperature & Pressure:Critical temperature: 548.00 K; Critical pressure: 4.5X10+6 Pa

